Laboratoires Grimberg,
l'expert des maux du quotidien pour toute la famille
A la une
Nos domaines d’expertise

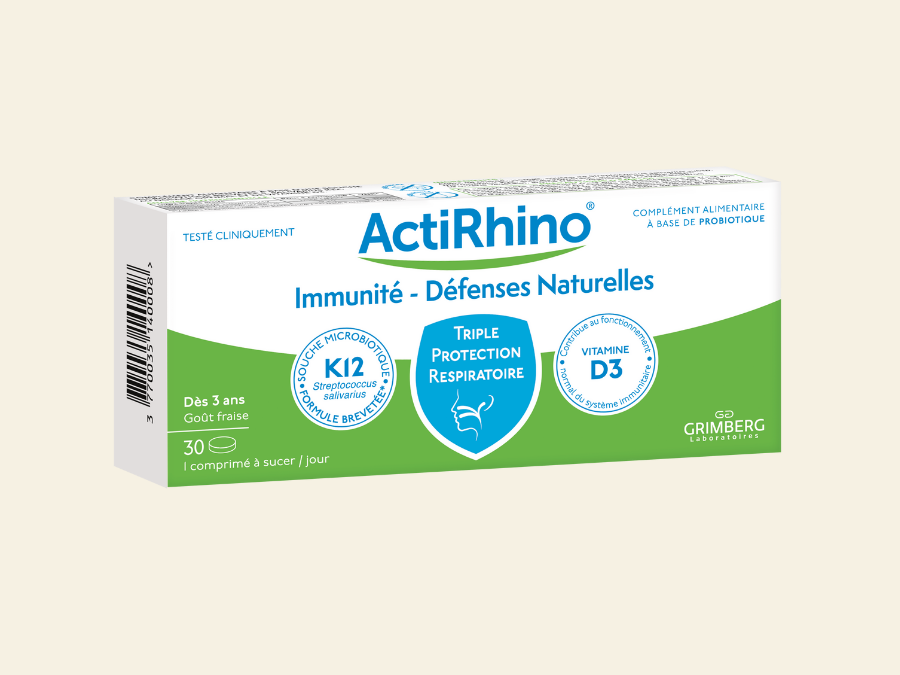
ORL
Rhume, rhinite, rhinopharyngite, otite
GASTRO ENTÉROLOGIE
Digestion difficile, ballonnements, reflux gastro-œsophagien, brûlures épigastriques

AUTRES SPÉCIALITÉS
Fatigue, irritabilité, anxiété, crampes, troubles du sommeil, ostéoporose
Nos solutions
Nos offres d’emploi
Envie de vivre une expérience marquante dans une entreprise à taille humaine vous donnant la possibilité de développer votre expertise ?
Nos métiers, d’une grande diversité : développement, production, logistique, médical, marketing, ventes, fonctions support… vous offrent autant de perspectives de carrières.
Rejoignez-nous !

Nos valeurs et notre engagement

INDÉPENDANCE
Un laboratoire familial, à taille humaine pour avoir la capacité d’agir vite, en toute maitrise et responsabilité

EXPERTISE
Une forte expertise dans le développement et la production de médicaments pour soulager les maux du quotidien

RIGUEUR PHARMACEUTIQUE
Une vigilance à tous les stades pour garantir des médicaments surs et de qualité
FABRICATION FRANÇAISE
Un attachement indéfectible au fabriqué en France

ÉTHIQUE ET CONFIANCE
Le respect d’une éthique rigoureuse pour développer une réelle relation de confiance avec nos patients et nos partenaires